THURSDAY, 24 JUNE 2021
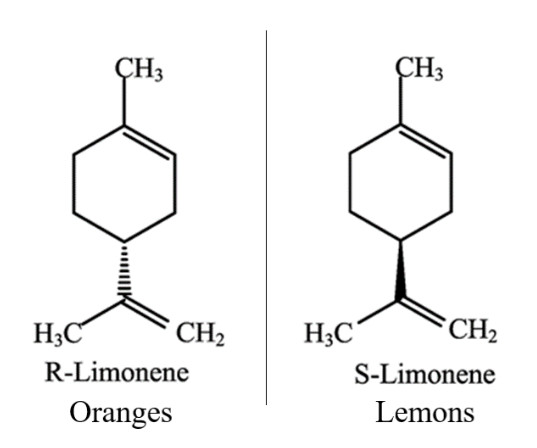
Enantiomers are molecular mirror images of each other. This means they have the same chemical composition, however their bond positions are reflected in such a way that the two molecules can no longer be superimposed. This can confer different biological properties to each molecule.These chemical differences can be experienced in the kitchen. One of the major essential oils in citrus fruit is limonene, which comes in 2 different enantiomers. The R form of limonene is responsible for the smell of oranges and the S form for lemons. One small change in positions of the atoms causes a drastic change in how we perceive the chemical. It’s worth giving them a sniff!
A more extreme example of the effect of enantiomers is thalidomide, a drug which was historically used to treat morning sickness. One thalidomide enantiomer causes birth defects and high infant mortality rates, whilst the other is an effective morning sickness treatment. Fair to say, birth defects were not observed in the clinical trials, but how could this happen? The problem arose because the molecule tested was obtained from biological sources and only contained one enantiomer; the one that treats morning sickness. However, when the drug was chemically synthesised, a racemic mixture was produced where it contained a 50:50 ratio of the different enantiomers. Whilst these chemicals have the same chemical composition, enzymes can distinguish the different enantiomers and so respond differently to them. Enantiomers present a big problem in terms of chemical synthesis, especially when making medicines. In some cases the dose has to be increased and in others the enantiomers must be separated or chemically converted into a single enantiomer. One tiny difference has the potential to cause dramatic change.
Oakem Kyne is a BlueSci contributor
