MONDAY, 3 FEBRUARY 2014
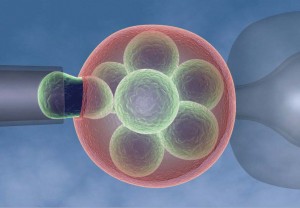
Imagine you wanted to have a child but had a serious genetic disorder that would be passed onto that child. What would you do?In 1989, a couple from the UK who were at risk of passing on a severe form of mental retardation on to their children became the first parents to use pre-implantation genetic diagnosis in order to avoid passing on this condition to their child. Although in vitro fertilisation – the technique behind ‘test tube babies’– had already been in use for 12 years at that time, it was the pre-implantation test performed by Professor Handyside and his team in the Royal Postgraduate Medical School in London that allowed for an early diagnosis and eliminated the need for termination of a pregnancy. In this diagnostic test, the embryos derived from the fertilisation procedure are grown for 2 to 3 days in the laboratory until they consist of 8 cells, of which one or two are removed. These cells are analysed to detect particular genetic abnormalities and the unaffected embryos transferred to the womb, where they are allowed to develop.
In the UK, pre-implantation genetic diagnosis is governed by the Human Fertilisation and Embryology Act 2008. While initially regulated on a case-by-case basis, since 2009 the Human Fertilisation and Embryology Authority has established a condition-by condition system whereby a clinic licensed for the procedure can carry out testing for any condition previously approved. In order for a particular condition to be approved for testing, this authority takes into consideration age of onset, the symptoms of the disease and their variability, the penetrance, availability, and efficacy of treatments. The approved conditions fall into three broad categories of genetic abnormalities: gene defects, chromosomal abnormalities and sex-linked conditions. Currently, pre-implantation genetic diagnosis tests for early onset Alzheimer’s disease, Cystic Fibrosis, Down’s syndrome, Haemophilia and Muscular Dystrophy, among others, totalling 263 conditions. This diagnostic test also tests for faulty genes which significantly increase the chance of an individual developing a serious disease. For instance, the BRCA1 gene, as its presence leads to a higher susceptibility to breast cancer. Another application of pre-implantation genetic diagnosis has been for cases of ‘saviour- sibling’ to ensure that the resulting child is a compatible donor for an existing sick sibling.
Pre-implantation genetic diagnosis raises a myriad of ethical and moral concerns. When dealing with an area of science involving the generation of human life, a heated discussion is bound to arise. Whilst the majority of the general public views pre-implantation genetic diagnostic as worthwhile and justifiable, if it alleviates suffering and can save lives, a minority considers it interference with nature and rejects this procedure. The most controversial facet of this procedure is its potential future use in creating ‘designer babies’. Embryos could be selected for certain physical and behavioural traits, which could lead to an attempt to establish a so-called ‘perfect’ society and bring about another form of eugenics. Unlike the UK, many countries lack any regulatory framework for this pre-implantation test. In the US 9 per cent of clinics are now offering embryo sex selection for non-medical reasons. In Sweden, gender selection has been legal since 2009. American consumer genetics company 23andMe was recently granted a patent that includes the possibility of embryo screening for physical traits, though they vehemently deny having any plans to explore this section of their patent.
Pre-implantation genetic diagnosis has helped many families and prevented children from suffering. But despite the beneficial impact this procedure has had, the question remains: who can stop these countries and companies from creating ‘designer babies’ on demand? The current absence of an answer may yet lead society down a very dangerous path.
Maria Mascarenhas is a 4th year PhD student in the Department of Haematology