SUNDAY, 7 NOVEMBER 2010
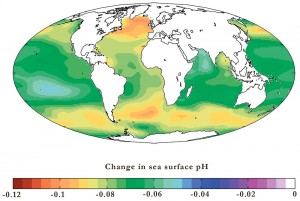
Life in the oceans is governed by a fascinating complexity of ecosystems and biogeochemical cycles that are at risk from a little talked about consequence of increasing atmospheric carbon dioxide: the acidification of the oceans. Approximately half of the carbon dioxide generated by humans since pre-industrial times has dissolved into the sea. The effects on marine life are still poorly understood, despite more than a decade of research, but if this acidification continues, it could cause damage that takes millennia to repair.Carbon dioxide is soluble in water, and when it dissolves, can react to produce carbonic acid and ions of bicarbonate and carbonate. The proton concentration in the solution rises, causing an increase in acidity. The dissolving of atmospheric carbon dioxide in this way is the origin of ocean acidification. Measurements in a wide range of locations over the last few decades have picked up increases in ocean acidity that correspond to increases in carbon dioxide concentrations in the atmosphere.
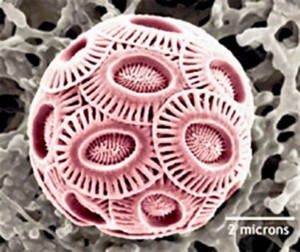
One of the most important consequences of acidification is its effect on calcifiers, marine organisms that construct shells and skeletal features from calcium carbonate. This involves the precipitation of carbonate ions through a range of mechanisms that are not understood in detail. However, the process commonly involves a step in which the organism isolates and de-acidifies seawater, to ease controlled crystallisation of carbonate. If the seawater is more acidic to begin with, this process requires more energy. Furthermore, calcium carbonate dissolves more easily at higher acidity, meaning that once structures have been formed, they will re-dissolve more easily and will be more difficult to maintain. Many small-scale studies of specific species, including corals, have shown a reduction in calcification rates and shell weights in response to increased carbon dioxide.
Corals can form vast yet intricate reef structures from carbonate and so are potentially threatened by acidification. Mainly formed in warm, shallow waters, reefs are incredibly important environments for many different reasons. Ecologically, they are hotspots of diversity, supporting countless species with their provision of food and shelter. Economically, they are worth tens of billions of pounds per year globally in income from tourism and fisheries, often for poorly developed tropical countries with few natural resources. Physically, they can act as important barriers to coastal erosion.
We do not fully understand reef ecosystems or their relationship with the wider ocean, so the implications of damage to reefs cannot be reliably predicted. In places where reefs have died naturally (for reasons unrelated to acidification) they are often replaced by thriving communities of algae and herbivorous fish. So life still goes on – but there is a dramatic decrease in diversity, and the variety of species present is different, with many commercially important species unable to survive.
Other calcifiers that are adversely affected by ocean acidification include a range of planktonic species that photosynthesise and are therefore primary producers at the base of the marine food web. While land plants tend to benefit from elevated carbon dioxide levels, these marine producers already process seawater to concentrate the gas, so they do not benefit from higher concentrations. However, they do suffer adverse effects from acidification: their growth is slowed and so their net productivity decreases, with knock-on effects throughout the oceanic ecosystem.
The effects on planktonic productivity are amplified by the fact that the majority of dissolved carbon dioxide is concentrated in the surface layer of the ocean. This layer – less than 200 metres deep – is well mixed and in close contact with the atmosphere, and therefore takes up carbon dioxide easily. It takes less than a decade for dissolved carbon dioxide to spread throughout the surface layer, but it takes hundreds to thousands of years for this layer to equilibrate with the deeper ocean. This property means that rapid human emissions have caused carbon dioxide to build up in the surface layer. Warming of the planet may also decrease vertical mixing, thus affecting the surface layer even more.
Plankton that photosynthesise live exclusively in the surface layer, since sunlight quickly diminishes with depth. They are therefore in the most acidic layer of the ocean, causing maximum impact on their productivity. The slow mixing of the surface layer with the deeper ocean means that significant damage could be done very rapidly, with a much longer time needed for recovery.
Acidification does not only vary with depth, but is also not evenly distributed across the globe. Carbon dioxide is more soluble at lower temperatures, so parts of the oceans at higher latitudes are affected more than warmer, more equatorial waters. The Southern Ocean surrounding Antarctica, which has a high concentration of photosynthesising primary producers, is of particular concern since its low temperatures make it more susceptible to acidification.
Elevated levels of carbon dioxide in the oceans may be harmful to non-calcifying organisms too, especially those with higher metabolism. Some experiments have shown detrimental effects on the health and reproductive ability of specific marine animals. However, the levels of carbon dioxide used in these experiments far exceed the most pessimistic predictions for human output, and it is unlikely that non-calcifiers will be directly harmed. Indirect consequences from the effects on plankton and other calcifiers could, however, be serious.
Fluctuations in the acidity of the ocean can be buffered to some extent by natural mechanisms: the dissolution of carbonate minerals in the sea decreases its acidity, and these are in plentiful supply. Ecosystems and species can also adapt and evolve to survive in the new conditions. But our rate of carbon dioxide output is unprecedented in the recent past and potentially too fast for these mechanisms to keep up. The carbonate buffer reactions certainly progress too slowly to significantly reduce the initial spike of acidity, and the physiological and chemical adaptations required of marine organisms may be too great to occur quickly enough.
The Palaeocene-Eocene Thermal Maximum that occurred 55 million years ago is a well-studied event that has several parallels to the current situation, and demonstrates the potential consequences of significant acidification. It was characterised by rapid output of carbon (probably as methane, which quickly oxidises to carbon dioxide in the atmosphere), comparable to the current human output. Sediment records from this time show a sharp warming event, the extinction of several sea floor calcifiers, and large-scale dissolution of sea floor carbonates; all of which are predicted outcomes of acidification. It took around 1,000 years for the climate to be perturbed, and 20,000 years to recover.
Ocean acidification caused by humans may not independently kill off marine organisms and ecosystems, but it will add significant stress that makes them more vulnerable to other factors such as climate change, pollution and large-scale fishing. Although this applies more to calcifiers than non-calcifiers, both will be affected.
Regardless of your opinion on global warming, the threat of ocean acidification is an independent and powerful motive to immediately reduce human carbon dioxide emissions. Though a lack of detailed understanding of the immensely complicated and inter-related systems involved makes exact predictions of the consequences impossible, the inevitable changes to the marine environment will surely impact heavily on Earth’s ecological stability, its biodiversity, and its nations’ bank balances.
Matthew Humphreys is a 4th year undergraduate in the Department of Earth Sciences