MONDAY, 4 JANUARY 2010
In 1911 the leading lights of theoretical physics clashed over their predictions of how conductive materials would behave at very low temperatures. Some believed that electrons would stand still in sufficiently cold conductors. Others thought they would move faster. The battle ended when Heike Kamerlingh Onnes, at the University of Leiden in Holland, discovered superconductivity.Usually, passing electricity through a conductor produces heat. Electrons collide with ions in the conducting material and with other electrons, and are scattered. The energy from these collisions is converted into heat and wasted. The higher the electrical resistance of the material, the more heat is produced.
In a superconductor, vibrations in the material cause the electrons to form pairs that are able to flow through the material without scattering. Below the critical temperature of a superconductor, which varies between materials, thermal motion cannot break up these pairs; the conductivity is perfect and no energy is wasted.
The critical temperatures of superconductors are close to ‘absolute zero’, the lowest possible temperature equivalent to -273 degrees centigrade. For example, niobium has the highest critical temperature of the elemental superconductors and still requires the temperature to be below -263.8 degrees centigrade.
Superconductivity remained something of a curiosity for most of the 20th century due to the low temperatures required. It was not until 1957 that a Nobel prize-winning theory of superconductivity was proposed that suitably explained all of the effects observed at the time. This theory predicted a maximum critical temperature of -243 degrees centigrade. Such temperatures are incredibly low compared with those encountered in everyday life – the coldest place on earth is only -80 degrees centigrade. Nevertheless, the properties of superconductors are so unique that they are used despite the low temperatures required.
Superconductors are used to create large magnetic fields. According to Ampere’s law, when a current flows it generates a magnetic field. With the ability to carry a high current density, superconducting materials therefore produce a very large magnetic field. The most notable use of this is in MRI scanners, in which a strong magnetic field is used to map the locations of water molecules in the brain and provide an accurate three-dimensional brain scan. Without superconductors, the space required to attain the necessary magnetic field would be prohibitive. Superconducting magnets are also used in the Large Hadron Collider, the particle accelerator used to study the smallest particles in the universe.
Alongside perfect conductivity, superconductors have another remarkable property. When placed in a magnetic field, they become magnetised themselves so as to exactly cancel the external field. The superconductor attains a north pole aligned with the north pole of the applied field, so they repel one another like two bar magnets aligned the wrong way. A superconductor placed above a large magnet will therefore levitate.
In Japan, lovers can be married on a floating magnetic plate, but the most commercial application of the levitation effect is the magnetic levitation (MAGLEV) train. These trains float above the track, and this greatly reduces the drag force. A MAGLEV connects Shanghai airport to the city and reaches four hundred kilometres per hour during the eight minute journey.
Although superconductors have already found applications, the hope of zero-resistance electrical cables at room temperature is the motivation behind much research into superconductivity. Currently, ten per cent of all electricity is wasted in transmission. This particularly affects renewable energy sources, because windy or sunny areas tend to be far from large populations. Superconducting cables would reduce this wastage.
Superconductors at room temperature could also create more efficient batteries. Since there is no resistance, a current flowing around a loop of superconductors will continue almost indefinitely (over 100,000 years according to calculations). Currently, we have to keep enough power plants running to maintain the peak load at all times, regardless of demand. Effective storage in batteries could allow significant reductions in the amount of power generated and would also help to balance generation from intermittent sources, such as solar power.
Although such room temperature superconductors are not yet available, a revolutionary series of new compounds were discovered in the late 1980s that display much higher critical temperatures. These materials, called cuprates, are based on a series of ceramic chemical compounds made up of copper oxide layers. Some of these materials superconduct at above -196 degrees centigrade, which is the boiling point of liquid nitrogen.
This discovery opened up new avenues for applications of superconductors, since nitrogen is much cheaper than lower temperature coolants like helium. In addition, the high critical temperature shows that the cause of superconductivity must be radically different to that proposed in the 1957 theory. High-temperature superconductivity is still not fully understood, but if the maximum critical temperature turns out to be much higher than previously thought, superconductors may be easier and cheaper to use in future.
Despite the promise of the cuprate ‘high-temperature’ superconductors, development has been slow. Among other problems, there are serious challenges in turning ceramic compounds into wires for transmission lines – imagine trying to make a tea cup into a cable. Consequently, the scientific community is constantly looking for new materials that display superconductivity.
Among other systems, carbon-60 Buckminster fullerene and graphite can be turned into carbon-based superconductors with the right addition of certain metals. Magnesium bromide was found to be a good superconductor only in 1999, despite having been well-known for over 100 years. A family of materials including certain uranium and plutonium compounds, called the ‘heavy-fermion’ superconductors, display a raft of interesting phenomena in which the electrons inside them appear to acquire an incredibly large mass when moving through the material.
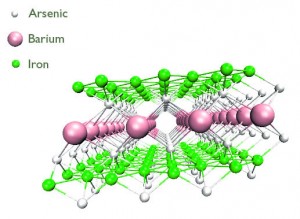
Research at the Cavendish Laboratory in Cambridge involves another family of superconductors, the iron arsenides. Discovered to be superconducting in early 2008, these materials have the second highest critical temperatures to the cuprates, promising new clues about the nature of superconductivity at high temperatures. Just like the cuprates, these materials are made into superconductors by ‘doping’ – the intentional introduction of impurities into a compound to change its structure and electrical properties. An alternative method, discovered at the Cavendish Laboratory, involves applying very large pressures, which makes these new materials superconduct at quite high temperatures. Ongoing research involves trying to prise apart the effect of pressure and doping on the electrical and structural properties in these materials in order to inform further theoretical discussion.
Superconductivity is perhaps the most spectacular scientific discovery yet to find a significant foothold in the real economy. But the increasing use of superconductivity in niche areas together with an active research community and a growing superconductor industry may provide a warmer future for this revolutionary phenomenon.
Jack Gillett is a PhD student in the Department of Physics