WEDNESDAY, 18 JANUARY 2012
How do you grow perfect crystals the size of a single human hair? In the case of proteins, the answer is often by painstaking trial and error.Proteins can form crystals just like other molecules when placed in the appropriate conditions. But proteins are fickle: each individual protein requires a unique and unpredictable set of conditions for successful growth. Many systems are set up simultaneously to explore the necessary conditions, varying parameters such as temperature, pH and the concentrations of protein and precipitant.
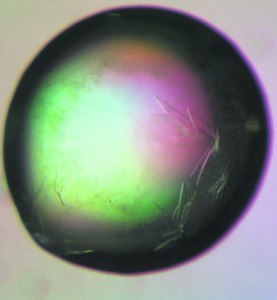
The method most commonly used for protein crystallisation is hanging drop vapour diffusion. This was the method used by Gengshi Chen, a third year Biochemistry student at Selwyn College, to grow the crystals that appear in the droplet shown in this issue’s cover image. In this technique a droplet containing purified protein, buffer and precipitant is placed on a glass slide. This is then hung over a reservoir of buffer and precipitant in higher concentrations to create a closed system. In order to attain equilibrium, water vapour slowly leaves the droplet and transfers to the reservoir. This causes the precipitant concentration in the droplet to increase to a level where crystallisation can occur.
But why do we need protein crystals? The aim of the crystallisation process is to grow a well-ordered crystal that is pure enough and large enough to be used for X-ray diffraction experiments. The resulting diffraction pattern can then be analysed to discern the structure of the protein.
The star-like aggregations in the cover image were observed approximately two days after initiating crystallisation. The final crystals, measuring just 50 to 100 micrometres across, were grown in five days. These were then mounted on nylon wire loops and stored in liquid nitrogen for transport to the European Synchrotron Radiation Facility (ESRF) in Grenoble for X-ray diffraction experiments.
Images of the protein crystals were also taken using polarised light microscopy as part of the Amgen Scholar Program at the Karolinska Institutet in Stockholm. This technique exploits a property of crystals known as birefringence. In birefringent materials, light travels at two different speeds depending on the direction in which it is travelling, and therefore splits into two separate beams when it enters the material. In polarised light microscopy, light passes through two polarising lenses oriented at right angles with the sample in between. If the sample is birefringent, it will rotate some of the light, allowing it to pass through the second polariser. The intensity of the transmitted light depends on the orientation of the crystal and this provides the necessary contrast to pick out the protein crystals in the droplet.
The particular protein under investigation in this case was a Class I major histocompatibility complex (MHC). Proteins of this class are found on virtually all cell types in the body and perform a vital role in the immune system by presenting short fragments of proteins from within the cell to white blood cells from outside. This allows the immune system to identify abnormal body cells, such as those infected with viruses or those that have turned malignant, and destroy them with white blood cells. Complexes formed by the MHC protein and foreign protein fragments with a more stable structure can survive longer on the cell surface, initiating a stronger immune response.
By growing crystals such as those in the cover image and analysing the structure of the protein, the relationship between the stability of these proteins and their molecular structure can be investigated. In the future, this could potentially lead to the design of tumour vaccines capable of inducing an immune response against cancer cells.
Lindsey Nield is a PhD student in the Department of Physics